Corrosion is defined as an attack on a material as a result of chemical, frequently electrochemical reaction, with the surrounding medium. According to this definition, the term corrosion can be applied to all materials, including non-metals. But in practice, the word corrosion is mainly used in conjunction with metallic materials.
Why do metals corrode? Apart from gold, platinum and a few others, metals do not occur in the nature in their pure form. They are normally chemically bound to other substances in ores, such as sulphides, oxides, etc. Energy must be expended (e.g. in a blast furnace) to extract the metals from the sulphides, oxides, etc to obtain pure metals.
Pure metals contain more bound energy, representing a higher energy state than that found in the nature as sulphides or oxides.
As all material in the universe strives to return to its lowest energy state, pure metals also strive to revert to their lowest energy state which they had as sulphides or oxides. One of the ways in which metals can revert to a low energy level is by corrosion. The products of corrosion of metals are often sulphides or oxides.
Chemical and electrochemical corrosion
Chemical corrosion can be seen as oxidation and occurs by the action of dry gases, often at high temperatures. Electrochemical corrosion on the other hand takes place by electrode reactions, often in humid environments, i.e. wet corrosion.
All metals in dry air are covered by a very thin layer of oxide, about 100Å (10-2µm) thick. This layer is built up by chemical corrosion with the oxygen in the air. At very high temperatures, the reaction with the oxygen in the air can continue without restraint and the metal will rapidly be transformed into an oxide.
At room temperature the reaction stops when the layer is thin. These thin layers of oxide can protect the metal against continued attack, e.g. in a water solution. In actual fact, it is these layers of oxide and/or products of corrosion formed on the surface of the metal that protect the metal from continued attack to a far greater extent that the corrosion resistance of the metal itself.
These layers of oxide may be more or less durable in water, for instance. We know that plain carbon steel corrodes faster in water than stainless steel. The difference depends on the composition and the penetrability of their respectively oxide layers. The following description of the corrosion phenomenon will only deal with electrochemical corrosion, i.e. wet corrosion.
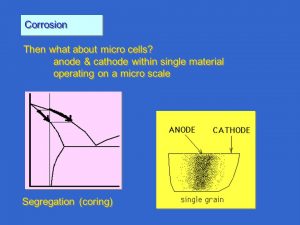
Corrosion cells
How do metals corrode in liquids? Let us illustrate this, using a corrosion phenomenon called bimetal corrosion or galvanic corrosion. The bimetal corrosion cell can e.g. consist of a steel plate and a copper plate in electrical contact with one another and immersed in an aqueous solution (electrolyte).
The electrolyte contains dissolved oxygen from the air and dissolved salt. If a lamp is connected between the steel plate and the copper plate, it will light up. This indicates that current is flowing between the metal plates. The copper will be the positive electrode and the steel will be the negative electrode.
The driving force of the current is the difference in electrical potential between the copper and the steel. The circuit must be closed and current will consequently flow in the liquid (electrolyte) from the steel plate to the copper plate. The flow of current takes place by the positively charged iron atoms (iron ions) leaving the steel plate and the steel plate corrodes.
The corroding metal surface is called the anode. Oxygen and water are consumed at the surface of the copper plate and hydroxyl ions (OH-), which are negatively charged, are formed. The negative hydroxyl ions “neutralize” the positively charged iron atoms. The iron and hydroxyl ions form ferrous hydroxide (rust).
In the corrosion cell described above, the copper metal is called the cathode. Both metal plates are referred to as electrodes and the definition of the anode and the cathode are given below.
Anode: Electrode from which positive current flows into an electrolyte.