Preparation of Oxygen
Oxygen is prepared in lab generally in two ways either by the application of heat or no application of heat.
Using heat:
Oxygen in lab is prepared by heating the mixture of powdered potassium chlorate and manganese dioxide in the ratio 4:1 in a hard glass test tube. The oxygen gas is observed in a gas jar through the downward displacement of water. The reaction involved is given below: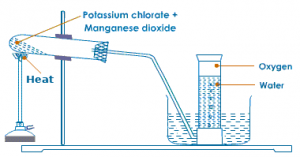
2KClO3−→−−−−−MnO2200−300∘C2KCl+3O2↑
Without heat:
The dry sodium peroxide is taken in a conical
2Na2O2+2H2O⟶4NaOH+O2↑
Physical properties of oxygen:
– Oxygen is colorless, odourless and tasteless gas.
– It is pale blue color in liquid and solid state.
– It is slightly soluble in water.
– It is heavier than air.
Chemical properties of oxygen:
Combustibility: It does not burn itself but it supports for combustion. Oxygen requires high initial heating due to its high bond dissociation energy of 493.4KJmol– between the O=O atoms.
Action with hydrogen: Oxygen when heated with hydrogen forms water.
2H2+O2−→Δ2H2O2H2+O2→Δ2H2O
Action with Nitrogen: Oxygen reacts with nitrogen at high temperature to give nitrogen dioxide.
N2+O2−→−−−3000∘C2NON2+O2→3000∘C2NO
NO+O2⟶2NO2NO+O2⟶2NO2
Action with carbon: Carbon when reacted with limited oxygen gives carbon monoxide and with excess oxygen, carbon gives carbon dioxide.
2C+O2limited⟶COCarbon Monoxide2C+O2limited⟶COCarbon Monoxide
2C+O2Excess⟶CO2Carbon Monoxide2C+O2Excess⟶CO2Carbon Monoxide
Action with ammonia: Oxygen reacts with ammonia to give nitric oxide and water. It is a reaction involved in manufacture of nitric acid in Ostwald’s process.
4NH3+5O2−→−−−Pt/MO800∘C4NO+6H2O↑4NH3+5O2→Pt/MO800∘C4NO+6H2O↑
Action with glucose: Glucose reacts with oxygen to give carbon dioxide, water and energy. This reaction takes place inside the human body.
Glucose + Oxygen→Carbon Dioxide + Water + EnergyGlucose + Oxygen→Carbon Dioxide + Water + Energy
i.e., C6H12O6+6O2⟶6CO2+6H2O+EnergyC6H12O6+6O2⟶6CO2+6H2O+Energy
Action with metals:
Oxygen combines with many metals to form their respective oxides.
4Na+O2−→−−−−−−−−−Room temperature2NaO24Na+O2→Room temperature2NaO2
2Na+O2−→−−300∘CNa2O22Na+O2→300∘CNa2O2
4K+O2⟶2K2O4K+O2⟶2K2O
2Mg+O2−→Δ2MgO2Mg+O2→Δ2MgO
2Zn+O2−→Δ2ZnO2Zn+O2→Δ2ZnO
4Al+3O2−→ΔAl2O34Al+3O2→ΔAl2O3
Action with iron: Oxygen when reacted with iron gives ferrous oxide. When excess of oxygen is passed, it
gives ferrosoferic oxide and further addition of oxygen gives ferric oxide. To be discusedFe+O2−→ΔFeOFerrousoxideFe+O2→ΔFeOFerrousoxide
6FeO+O2−→Δ2Fe3O4Ferrosofericoxide6FeO+O2→Δ2Fe3O4Ferrosofericoxide
4Fe+3O2−→Δ2Fe3O3Ferricoxide4Fe+3O2→Δ2Fe3O3Ferricoxide
When oxygen is reacted with oxygen in presence of water, rust is formed.
4Fe+3O2+2H2O⟶2Fe2O3⋅XH2ORust4Fe+3O2+2H2O⟶2Fe2O3⋅XH2ORust
Uses of oxygen:
– It is used for artificial respiration in hospitals, mountaineers in high altitude, miners and sea divers in the form of oxygen mask.
– It is used as aero fuel in rocket engines and planes.
– It is used for the generation of energy inside our body.
– It is used as strong oxidizing agent in laboratory.
– It is used by the plants for the process of photosynthesis.
– It is used in preparing different explosives.
– It is used as a germicides and insecticides.
– It is the main element for the formation of ozone.